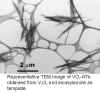



Vanadium oxide nanotubes (VOx-NTs) were obtained as the main product in a sol-gel reaction followed by hydrothermal treatment (7d at 180°C) from different vanadium oxide precursors [V2O5, VOCl3, VO(OiPr)3, HVO3] and primary amines or diamines.
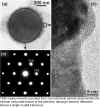
Transmission electron microscopy (TEM) investigations performed along the longitudinal projection direction show that the product consists exclusively of multiwalled nanotubes. The walls comprise between 2 and 30 parallel layers. The outer diameters range from 15 to 100 nm, the inner diameters from 5 to 50 nm. The lengths vary from 0.5 mm to a maximum of 15 mm. Predominantly open tubes are observed.
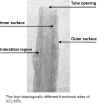
Cross-sectional TEM images reveal that the majority of the tubes shows a scroll-like morphology instead of concentric circular cylinders.
XRD patterns show the highly intense and sharp 00l reflections that are typical for a well-ordered layered structure at low scattering angles. The inter-layer distances of these reflections range from 1.7 to 3.8 nm and correlate linearly with the template size. This indicates that the template molecules are located in between the vanadium oxide layers. At higher scattering angles, less intense and broad hk0 reflections appear, which can be indexed on the basis of a square lattice with a = 0.61 nm. Their positions are not influenced by the template size.
According to elemental analysis and magnetic measurements, the elemental composition of vanadium oxide nanotubes can be expressed by the general formula [CnH2n+1NH2]1.9V7O17 for monoamines with n = 12-18.
F. Krumeich, H.-J. Muhr, M. Niederberger, F. Bieri, B. Schnyder, R. Nesper
J. Am. Chem. Soc. 1999, 121, 8324
Morphology and Topochemical Reactions of Novel Vanadium Oxide Nanotubes
----------------------------------
H.-J. Muhr, F. Krumeich, U. P. Schönholzer, F. Bieri, M. Niederberger, L. J. Gauckler, R. Nesper
Adv. Mater. 2000, 12, 231
Vanadium Oxide Nanotubes - a New Flexible Vanadate Nanophase
----------------------------------
F. Krumeich, H.-J. Muhr, M. Niederberger, F. Bieri, M. Reinoso, R. Nesper
Nanophase and Nanocomposite Materials III, MRS Symp. Proc. 2000, 581, 393
Vanadium Oxide Nanotubes with Diamine Templates
----------------------------------
M. Niederberger, H.-J. Muhr, F. Krumeich, F. Bieri, D. Günther, R. Nesper
Chem. Mater. 2000, 12, 1995
Low-Cost Synthesis of Vanadium Oxide Nanotubes via Two Novel Non-Alkoxide Routes
----------------------------------
F. Krumeich, H.-J. Muhr, M. Niederberger, F. Bieri, R. Nesper
Z. anorg. allg. Chem. 2000, 626, 2208
The Cross-Sectional Structure of Vanadium Oxide Nanotubes Studied by Transmission Electron Microscopy and Electron Spectroscopic Imaging
----------------------------------
Krishnan S. Pillai, F. Krumeich, H.-J. Muhr, M. Niederberger, R. Nesper
Solid State Ionics 2001, 141-142, 185
The First Oxide Nanotubes with Alternating Inter-Layer Distances